INTRODUCTION
Clinical Diagnostic Laboratory (CDL) is one of the services located at Pusat Perubatan USM Bertam (PPUSMB), Universiti Sains Malaysia, Bertam, Kepala Batas, Pulau Pinang.
Services are offered to government and private hospitals / clinics / laboratories. This manual emphasizes the importance of proper pre-analytical procedures regarding the collection of specimens, correct usage of specimen containers and proper transportation to ensure efficient, effective, precise and accurate tests and delivery of results within the acceptable time interval.
OBJECTIVES
CDL provides medical laboratory services in Integration Lab (Haematology, Chemical Pathology and Immunology) Genetics, Histopathology and Cytology, Microbiology, Transfusion Medicine, Molecular Diagnostics. Our customers include AMDI wards / clinics, government hospitals / clinics / laboratories and private hospitals / clinics / laboratories. To achieve quality services in line with international practice, CDL is committed to:
- providing professional services, ensuring accurate and precise results in accordance with international standard methodologies,
- implementing a quality management system, which is compiled to MS ISO 15189 so as to ensure management plans and technical operations are established and controlled,
- ensuring all personnel who are involved in examination activities are familiar with the Laboratory Quality Management System (LQMS), and implement the possible process at all the time, and
- ensuring continual improvement of its LQMS.
ORGANIZATION CHART AND VARIOUS UNITS
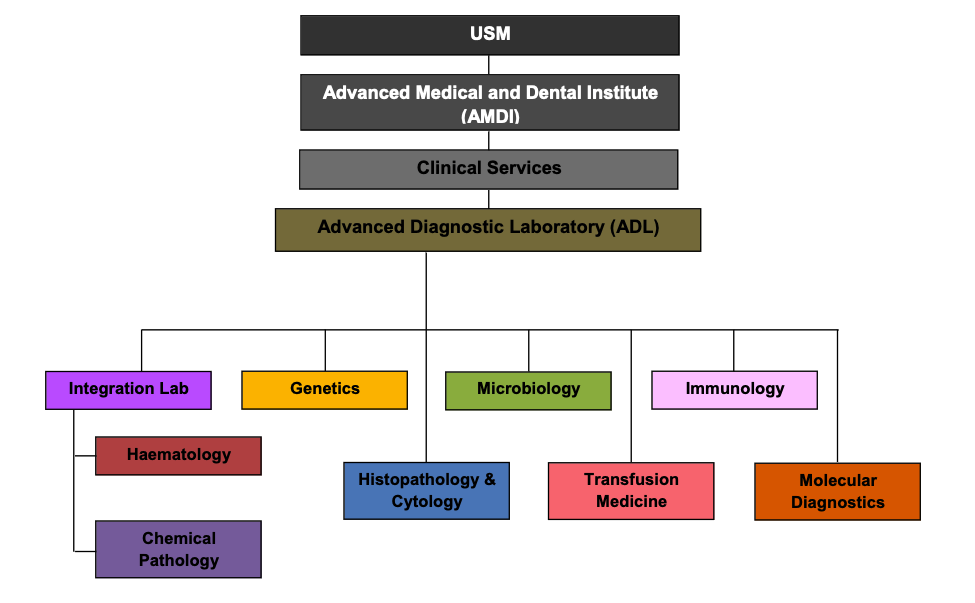
The laboratory services include:
- Chemical Pathology
- Histopathology and Cytology* c) Genetics
- Molecular Diagnostics*
- Haematology
- TransfusionMedicine
- Immunology*
- Microbiology
Note: Cytology, Molecular Diagnostic and Immunology services are not yet MS ISO 15189 accredited.
WHO TO CONTACTS
Clinical Diagnostic Laboratory (CDL),
Pusat Perubatan USM Bertam,
Universiti Sains Malaysia,
13200 Kepala Batas,
Pulau Pinang.
AMDI Operator: 04-562 2888
Direct line: 04-562 XXXX*
*Extension:
Receiving counter - 2711
Integrated Unit (Haematology) - 2693
Integrated Unit (Chemical Pathology) - 2686
Integrated Unit (Immunology) - 2331
Genetics - 2692
Histopathology and Cytology - 2685
Microbiology - 2696
Transfusion Medicine - 2700
Molecular Diagnostics** - 2293
**Note: Molecular Diagnostics Unit is located at Animal Research Complex (ARC), IPPT.
BUSINESS HOURS
Schedule for receiving specimen:
All specimens must reach the ADL receiving counter.

After office hours / public holiday all the request shall be sent directly to the specific unit (Chemical Pathology, Haematology, Transfusion Medicine and Microbiology).
*Note:
- For specimen involving Genetics Unit & Immunology Unit, clients should make appointments and samples should reach ADL receiving counter before 3.00 pm except for urgent samples.
- For specimen involves Immunology Unit, clients should make a contact to the Immunology Laboratory to make appointment. Preferably patient’s sample should be arrived before 10.00 am unless agreed otherwise.
SAMPLE SUBMISSION REQUIREMENTS
CDL only receive samples from requesting parties.
All samples should be sent directly to the CDL receiving counter.
All samples should be treated as biohazards and to be dispatched to CDL as soon as possible.
Please refer specific requirements for samples requiring special transportation procedures.
The request form should be completely and correctly filled manually or equivalent. Samples and request forms should be sent to CDL receiving counter. Details of patient must include the following information:
- Name
- Identification Card No.
- Age / Date of birth
- Gender
- Location / contact details of the patient
- Clinical summary
- Date and time of sample collection
- Type of sample and test requested
- Name and signature of the doctor requesting the test and stamp
For internal request (within PPUSMB), all request shall be made using Hospital Information System, Care2X. Please note that some tests require hardcopy request form. Please refer to the requirements of each laboratory.
URGENT REQUESTS
Clients need to inform CDL via phone before sending urgent samples (except for Immunology Unit and Histopathology and Cytology Unit). Urgent samples must reach CDL counter as soon as possible and should be labelled ‘URGENT’ or equivalent, with date and time of request clearly stated.
Requests which fail to adhere to the above instructions will not be entertained as ‘URGENT’ or equivalent.
For list of urgent request, please refer to each specific unit.
PANIC VALUE
Panic value is defined as any result outside the normal ranges to a degree that may pose immediate health risk to the individuals or require immediate action on the part of the treating doctors. It is the responsibility of the laboratory to immediately NOTIFY the doctor of the panic values.
ADL WORKFLOW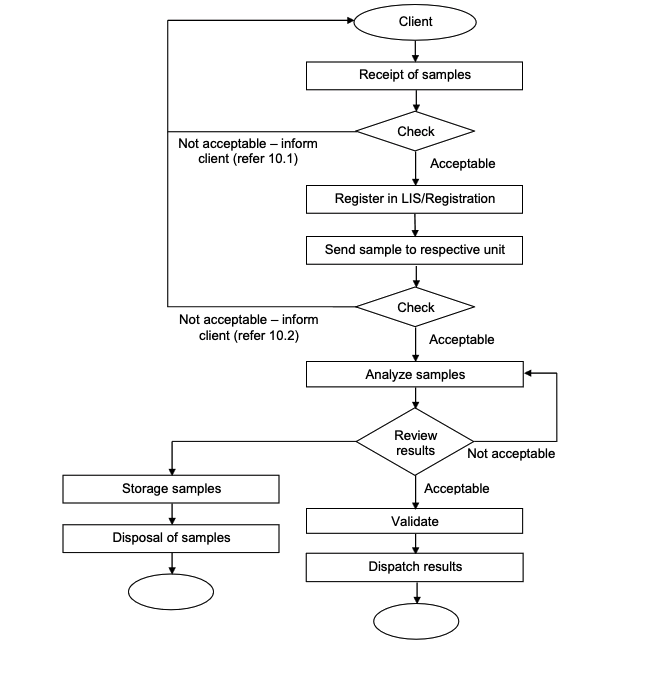
REJECTION CRITERIA
- First level rejection criteria - samples shall not be accepted or processed if any of the following criteria exist
- No name on the request form or electronic form (LIS) / specimen.
- Information on request form or electronic form (LIS) is not tally with specimen.
- Incomplete identification card / registration number.
- No name, signature and stamp of authorized requester / clinician.
- Test is not indicated.
- Unsatisfactory specimen, for example spillages, breakages and etc.
- Second level rejection criteria – sample not suitable to process if the following criteria exist:
- Inappropriate sample container / request form.
- Compromised sample such as haemolysed, clotted, insufficient, aged or other causes of unsuitable sample for analysis.|
- Test is not available.
- Inappropriate sample transportation.
- No clinical history information (whenever applicable).
VERBAL REQUESTS
CDL accepts verbal requests for additional tests by calling or informing the respective units by the requesting medical practitioner followed by request form or electronic equivalent within a given time. Acceptance criteria for verbal requests are stated in the user manual for each unit.
UNDERSTANDING OF EXAMINATION PROCESSES (TEST METHODOLOGY)
The users are expected to understand the general principles of examination processes for the test results generated in CDL. A detail description of examination processes will be provided upon request.
SPECIMEN COLLECTION & LABELLING
- Specimen is to be collected in the ward / clinics / others. Please refer to specific unit / test for proper specimen collection procedure (where indicated).
- Clear instruction is to be given to the patient if the specimen needs to be collected by the patient.
- All specimens from the patients are to be put in biohazard bag and attached with the request forms.
- All specimens shall be treated as biohazard and to be dispatched to the laboratory as soon as possible. (Please refer to the specific test that requires special transportation procedures in each unit).
- Formultiple specimen collection, please follow “order of draw” rules.
- Each specimen shall have a label firmly attached to the specimen container and bearing the following information:
- Patient’s name
- Patient’s registration number (PID)
- Name of ward or clinic
- Type of specimen (including specific anatomic site)
- Date and time of specimen collection
CONSENT
Patient consent is required where referral is needed (e.g. consent to disclose clinical information and family history to relevant healthcare professionals).
KNOWN FACTORS THAT AFFECT THE PERFORMANCE OF THE EXAMINATION / INTERPRETATION OF THE RESULT
- Clotted sample
- Lysed sample
- Lipemic sample
- Aged sample
- Icteric sample
- Contaminated sample
- Insufficient sample
- Wrong container
- Inappropriate sample transportation
- Insufficient fixative / wrong fixative
CONFIDENTIALITY
All tests done by CDL, PPUSMB are confidential between CDL and customers. Personnel are not allowed to disclose any information that can destitute the safety and confidential of test work.
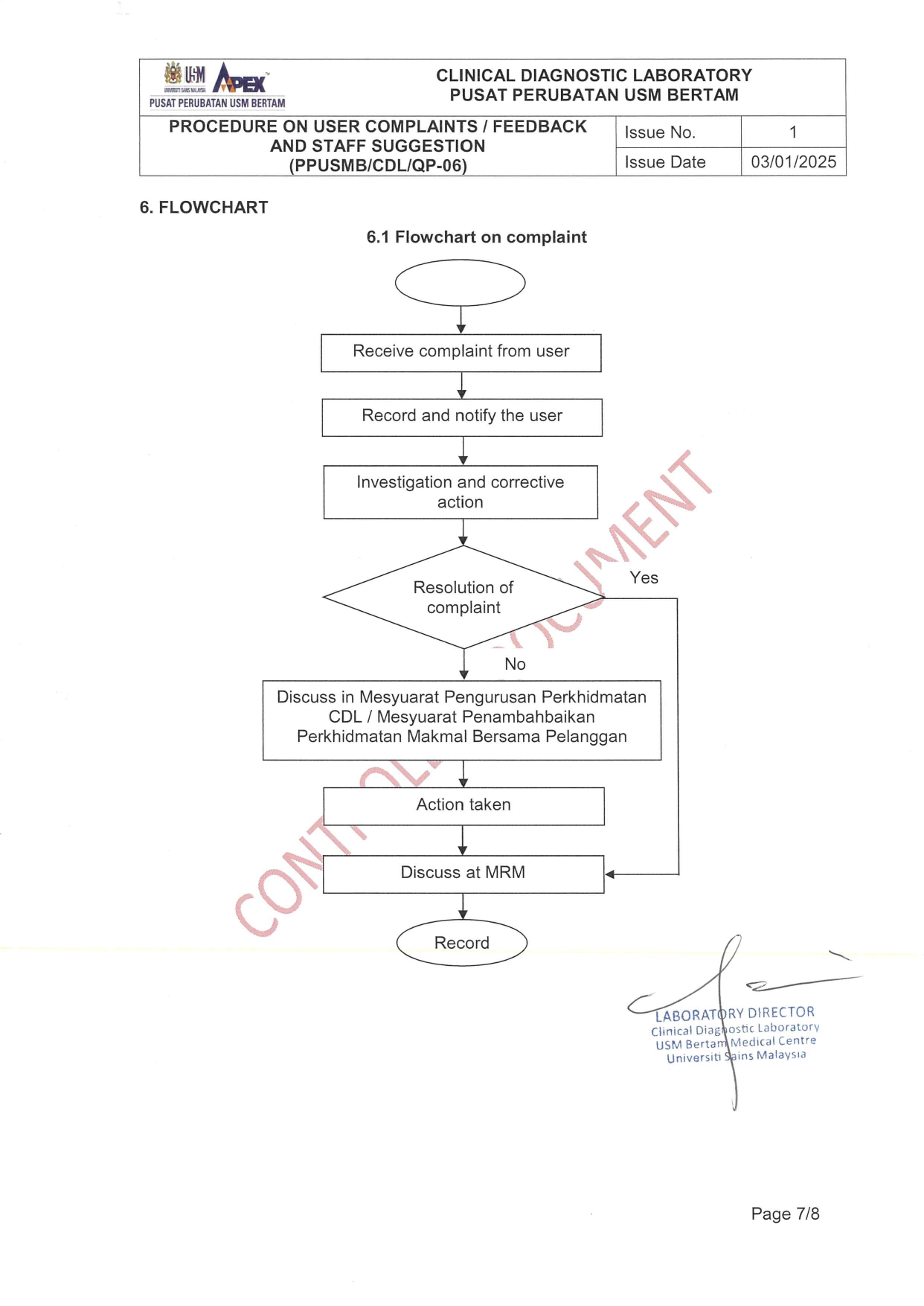
CUSTOMER COMPLAINTS AND FEEDBACK
Any complaints, compliments or feedback can be directly addressed to CDL through verbal or written. Verbal complaint can be done through phone or directly to the staff while written complaint via complaint form, formal letter or email (
Customer Complaint Form (ADL/QP6/F-1) can be downloaded from CDL website.
FURTHER INQUIRIES
Further Inquiries regarding this user manual could be directed to:
Quality Manager
Phone: 04-562 2677
Email: